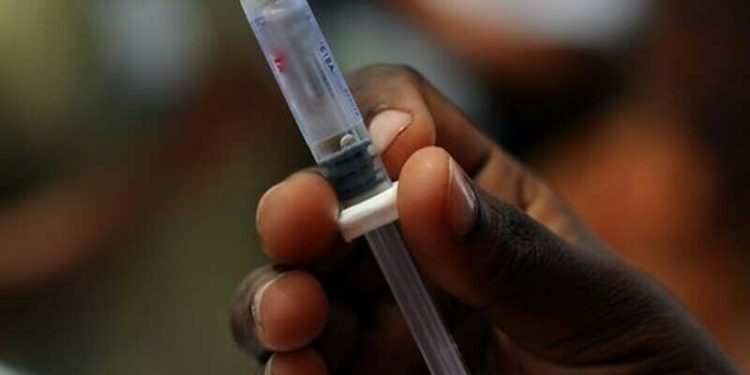
The Drug Regulatory Authority of Pakistan (Drap) on Tuesday directed three pharmaceutical companies to recall “substandard” medical devices from the market and advised pharmacists and chemists to stop supplying these products.
“[The] Central Drugs Laboratory Karachi informed the Drug Regulatory Authority of Pakistan that the samples of the below-mentioned medical devices have been declared as ‘Substandard’,” an alert issued by Drap stated.
The alert identified Zindagi Auto Disable Syringe 5ml, the Ultra Fine SMD Painless Syringe 5ml and Ultra Fine SMD Painless Syringe 3ml as the substandard devices.
“Use of these syringes, in invasive or intravenous procedures, poses a significant risk of introducing microbial contaminants into the patient’s body, which may result in localised infections, abscesses, or life-threatening systemic infections, particularly in immunocompromised individuals,” Drap said.
“All pharmacists and chemists working at distribution and pharmacies should immediately check their stocks and stop supplying the mentioned products,” the statement said, adding that the remaining stocks should be quarantined and returned to the supplier or the company.
width=”100%” frameborder=”0″ scrolling=”no” style=”height:250px;position:relative”
src=”
sandbox=”allow-same-origin allow-scripts allow-popups allow-modals allow-forms”>
The Zindagi Auto Disable Syringe was declared “substandard” on the basis
of a sterility test, while the Ultra Fine SMD Painless Syringes were declared “substandard” on the basis of a sterility test and description test with “clear visible black particles found in the barrel of the syringe”.
The alert advised consumers to stop using products bearing the affected batch number and contact their physician or healthcare provider if they experienced any problems that may be related to using the items. It further urged them to report the incident to Drap.
Last year in March, Drap directed a pharmaceutical company to recall a syrup, which is given to children to treat fever, from the market and advised health professionals not to prescribe it.
In January 2024, Drap directed pharmaceutical companies to recall nine contaminated syrups, according to its chief executive officer.